Semenkovich Lab
The Semenkovich lab is interested in lipid metabolism and how it promotes the complications of diabetes, obesity, and insulin resistance. The work is translational, spanning cultured cells, animal models and humans. Lipid metabolism is important for many disease complications. Specific lipid molecules like palmitate are normally attached to a considerable number of proteins. We have engineered mice with a diminished capacity to remove the fatty acid palmitate from proteins in several tissues. These mice are models for important diabetes complications like peripheral artery disease and uncontrolled diabetes. We have also generated mice with overproduction of fatty acids in several tissues. These different models are being studied in parallel with humans that have diabetes, obesity, and their complications in an effort to develop new therapies that can decrease the suffering of people with metabolic diseases.
Principal Investigator

Clay F. Semenkovich, MD
Chief, Division of Endocrinology, Metabolism and Lipid Research
- Phone: 314-362-3500
- Fax: 314-747-3963
- Email: csemenko@nospam.wustl.edu
Irene E. and Michael M. Karl Professor
Director, Diabetes Research Center
Professor of Medicine and of Cell Biology and Physiology
Team Members
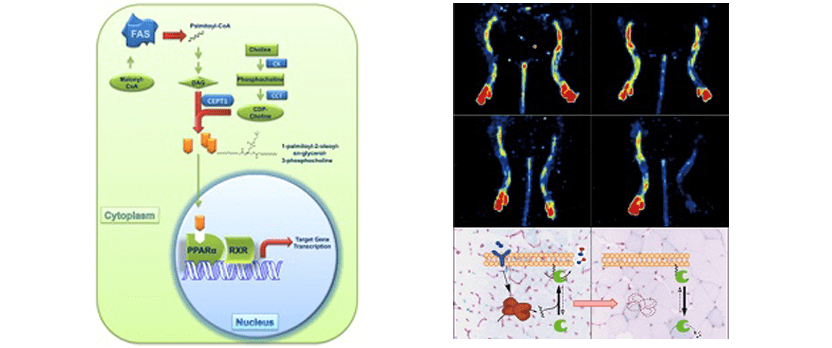
Skeletal muscle lipid flux: running water carries no poison.
Funai K, Semenkovich CF.
AJP – Endo. 2011 August.
PMCID: PMC3275151
Link to Publication
De Novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation.
Wei X, Schneider JG, Shenouda SM, Lee A, Towler DA, Chakravarthy MV, Vita JA, Semenkovich CF.
J Biol Chem. 2010 Nov 23.
PMID: 21098489
Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter.
Lodhi IJ, Wei X, Semenkovich CF.
Trends Endocrinol Metab. 2010 Oct 1.
PMID: 20889351
Deletion of Tis7 protects mice from high-fat diet-induced weight gain and blunts the intestinal adaptive response postresection.
Yu C, Jiang S, Lu J, Coughlin CC, Wang Y, Swietlicki EA, Wang L, Vietor I, Huber LA, Cikes D, Coleman T, Xie Y, Semenkovich CF, Davidson NO, Levin MS, Rubin DC.
J Nutr. 2010 Nov;140(11):1907-14. Epub 2010 Sep 22.
PMID: 20861213
Genetic, epigenetic, and gene-by-diet interaction effects underlie variation in serum lipids in a LG/JxSM/J murine model.
Lawson HA, Zelle KM, Fawcett GL, Wang B, Pletscher LS, Maxwell TJ, Ehrich TH, Kenney-Hunt JP, Wolf JB, Semenkovich CF, Cheverud JM.
J Lipid Res. 2010 Oct;51(10):2976-84. Epub 2010 Jul 2.
PMID: 20601649
Diet-Dependent Genetic and Genomic Imprinting Effects on Obesity in Mice.
Cheverud JM, Lawson HA, Fawcett GL, Wang B, Pletscher LS, R Fox A, Maxwell TJ, Ehrich TH, Kenney-Hunt JP, Wolf JB, Semenkovich CF.
Obesity (Silver Spring). 2010 Jun 10.
PMID: 20539295
Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis.
Schneider JG, Yang Z, Chakravarthy MV, Lodhi IJ, Wei X, Turk J, Semenkovich CF.
J Biol Chem. 2010 Jul 23;285(30):23398-409. Epub 2010 May 17.
PMID: 20479009
Calpain-10 is a component of the obesity-related quantitative trait locus Adip1.
Cheverud JM, Fawcett GL, Jarvis JP, Norgard EA, Pavlicev M, Pletscher LS, Polonsky KS, Ye H, Bell GI, Semenkovich CF.
J Lipid Res. 2010 May;51(5):907-13.
PMID: 20388922
p53 is required for chloroquine-induced atheroprotection but not insulin sensitization.
Razani B, Feng C, Semenkovich CF.
J Lipid Res. 2010 Jul;51(7):1738-46. Epub 2010 Mar 5.
PMID: 20208057
Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet.
Song H, Wohltmann M, Bao S, Ladenson JH, Semenkovich CF, Turk J.
Am J Physiol Endocrinol Metab. 2010 Jun;298(6):E1097-114. Epub 2010 Feb 23.
PMID: 20179248
Identification of a physiologically relevant endogenous ligand for PPARalpha in liver.
Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF.
Cell. 2009 Aug 7;138(3):476-88. Epub 2009 Jul 30.
PMID: 19646743
Calpain-10 is a component of the obesity-related quantitative trait locus, Adip1.
Cheverud JM, Fawcett GL, Jarvis JP, Norgard EA, Pavlicev M, Pletscher LS, Polonsky KS, Ye H, Bell GI, Semenkovich CF.
J Lipid Res. 2009 May 12.
PMID: 19436065
Getting away from glucose: stop sugarcoating diabetes.
Razani B, Semenkovich CF.
Nat Med. 2009 Apr;15(4):372-3. No abstract available.
PMID: 19350008
Why we should put clothes on mice.
Lodhi IJ, Semenkovich CF.
Cell Metab. 2009 Feb;9(2):111-2.
PMID: 19187768
Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation.
Chakravarthy MV, Zhu Y, Yin L, Coleman T, Pappan KL, Marshall CA, McDaniel ML, Semenkovich CF.
J Lipid Res. 2009 Apr;50(4):630-40. Epub 2008 Nov 22.
PMID: 19029118
Insulin resistance and atherosclerosis.
Razani B, Chakravarthy MV, Semenkovich CF.
Endocrinol Metab Clin North Am. 2008 Sep;37(3):603-21, viii. Review.
PMID: 18775354
Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models.
Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, Graham MJ, Davidson NO.
Hepatology. 2008 Oct;48(4):1097-105.
PMID: 18697204
Requirement for p38 mitogen-activated protein kinase activity in neointima formation after vascular injury.
Proctor BM, Jin X, Lupu TS, Muglia LJ, Semenkovich CF, Muslin AJ.
Circulation. 2008 Aug 5;118(6):658-66. Epub 2008 Jul 21.
PMID: 18645058
You’ve reached Dr. Semenkovich’s lab — Patients should visit the Washington University Physicians website.
Students, staff, and faculty interested in positions should visit the Division’s website.
Lab Contact Information
Clay F. Semenkovich, M.D.
Irene E. and Michael M. Karl Professor
Chief, Division of Endocrinology, Metabolism & Lipid Research
Professor of Medicine and of Cell Biology and Physiology
Mailing Address
660 S. Euclid Ave
Campus Box 8127
St. Louis, MO 63110
Phones & Faxes
Office: (314) 362-7617
Laboratory: (314) 747-8282
Fax: (314) 362-7641
Office Location: 842 SW Tower
Patients
See contact information at the Washington University Physicians website.




