Stay up to date on the latest research and division news by signing up for our newsletter, here.

Bambouskova Lab
Our lab studies metabolic pathways that support and regulate immune cell activation. We focus on the function of metabolites and cellular metabolic processes that interplay with inflammatory signaling in immune cells, particularly in macrophages. We are also interested in how systemic metabolism affects immune cell functions in the context of obesity and the onset of T2D. Our lab uses molecular biology and biochemistry techniques together with systems approaches to tackle crosstalk between metabolism and immune cell signaling to better understand mechanisms underlying inflammatory diseases.

Bernal-Mizrachi Lab
The long-term goal of our laboratory is to determine the mechanisms responsible for metabolic syndrome and its vascular complications. We are particularly interested in understanding the mechanisms of the interactions between vitamin D signal transduction, intracellular calcium, and endoplasmic reticulum stress in critical tissues responsible for the development of metabolic syndrome and its complications.
Crewe Lab
The Crewe lab uses transgenic mouse lines, cell culture and biochemistry to understand extracellular vesicle (EV)-mediated signaling during homeostatic and pathologic metabolic regulation. Our work has uncovered the existence of an expansive EV-mediated signaling network within the adipose tissue proper, and from the adipose tissue to other organs. This mostly adipocyte-derived EV population consists of exosome-like vesicles that carry proteins, RNAs and lipid species that can modulate a variety of signaling pathways in recipient cells. The focus of the lab is to determine how the various cargo of adipocyte EVs signal within the adipose tissue and from the adipose tissue to distal organs to modulate metabolism in obesity.

Hughes Lab
We are an islet biology lab focusing on primary cilia. Our lab has generated new models to study the role of cilia in signal transduction, hormone secretion, and cell-cell communication. We use multi-disciplinary approaches and collaborate widely on campus. Core techniques include molecular biology, in vivo metabolic studies, light and electron microscopy, proteomics, gene expression profiling.

Lodhi Lab
Our laboratory takes an integrative approach to study the biology of peroxisomes in the context of metabolic disorders, such as obesity and diabetes. Peroxisomes are intimately associated with lipid droplets and mitochondria. Their ability to carry out fatty acid oxidation and lipid synthesis, especially the production of ether-linked phospholipids, may be critical for generating cellular signals required for normal physiology.
Millman Lab
The Millman laboratory is focused on the generation insulin-producing pancreatic β cells for the study and treatment of diabetes. EXPERTISE: We specialize in the application of a wide range of state-of-the-art technologies to discover a cure for diabetes. Visit the Millman lab website at: https://sites.wustl.edu/millmanlab/

Muegge Lab
Our lab studies the intestinal endocrine system with the goal of finding new treatments for metabolic and intestinal diseases. Enteroendocrine cells (EECs) in the intestine make more than 20 different hormones, but this system isn’t well understood because the cells are rare, diverse, and historically hard to grow in the lab. We developed a new experimental model that overcomes this barrier to culture and study EECs from primary mouse and human intestinal stem cells. We are using these models to understand the transcriptional and epigenetic control of intestinal and EEC development in health and metabolic disease. Our research team merges techniques from developmental biology, genomics, and systems biology in a highly interdisciplinary, collaborative, and supportive environment.

Remedi Lab
The major focus of the Remedi laboratory is to study in vivo physiology in various mouse models of diabetes to unravel the underlying mechanisms of pancreatic β-cell failure in glucotoxic stages, and their consequences in both pancreatic and extra-pancreatic tissues. Development of secondary loss of β-cell mass and antidiabetic drug sensitivity in long-standing diabetic patients is not completely understood. Recently, much attention has been gathered on cell plasticity challenging the current paradigms of how diabetes progresses.

Semenkovich Lab
The Semenkovich lab is interested in lipid metabolism and how it promotes the complications of diabetes, obesity, and insulin resistance. The work is translational, spanning cultured cells, animal models and humans. Lipid metabolism is important for many disease complications. Specific lipid molecules like palmitate are normally attached to a considerable number of proteins. We have engineered mice with a diminished capacity to remove the fatty acid palmitate from proteins in several tissues. These mice are models for important diabetes complications like peripheral artery disease and uncontrolled diabetes. We have also generated mice with overproduction of fatty acids in several tissues. These different models are being studied in parallel with humans that have diabetes, obesity, and their complications in an effort to develop new therapies that can decrease the suffering of people with metabolic diseases.
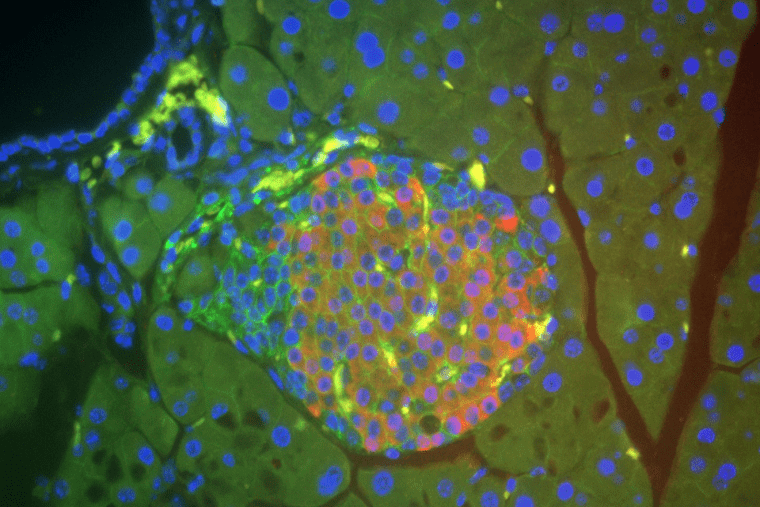
Urano Lab
The ultimate goal of our research is to find a cure for Wolfram syndrome, a prototype endoplasmic reticulum disease. Wolfram syndrome is a rare autosomal recessive genetic disorder with clinical signs apparent in early childhood. This condition is characterized by juvenile-onset diabetes, optic nerve atrophy, deafness, diabetes insipidus and neurodegeneration, and it may result in death in middle adulthood.