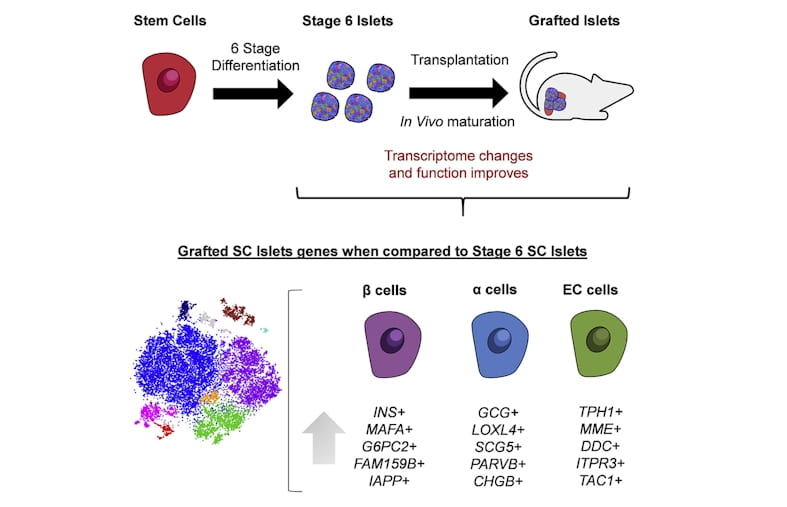
Researchers at Washington University have been generating insulin-producing islets in the laboratory from human stem cells. This approach promises a virtually unlimited supply of replacement insulin-producing tissues to functionally cure patients with diabetes. However, cell-derived islets are immature compared to islets from the body, lacking the expression of genes associated with maturation.
Here, a team of researchers from the laboratory of Dr. Jeffrey Millman at Washington University in St. Louis have discovered that these stem cell-derived islets are capable of maturing after transplantation to resemble real islets more closely from humans. The team, with co-first authors Punn Augsornworawat and Kristina Maxwell, accomplished this feat by developing a new methodology to isolate transplanted cells and performed single-cell RNA sequencing to catalogue these changes in individual cells.
The mice used in the experiments had severe diabetes. After several months, these mice were completely cured of diabetes, with the transplanted stem cell derived islets providing robust insulin release and blood glucose regulation, equivalent to healthy mice.
These long-term improvements in function are associated with increased expression of mature genes, such as MAFA and G6PC2. In addition, the team also identified other mature markers including FAM159B, and MT1X, to robustly confirm maturation and found that secretion of the peptide IAPP to be a detectable biomarker for maturation more robustly.
These findings highlight the similarities between lab-grown islets after transplantation and native islets from the body and provides a new technological pipeline allowing for more rigorous study of cellular maturation of differentiated cells from stem cells. The research article was published on August 25, 2020 in the journal of Cell Reports (https://doi.org/10.1016/j.celrep.2020.108067).
This study was supported by the National Institutes of Health (5R01DK114233, T32DK108742, P30CA91842, UL1TR000448), a JDRF Career Development Award (5-CDA-2017-391-A-N), Washington University-Centene Personalized Medicine Initiative, David and Deborah Winston Fellowship in Diabetes Research, and joint funds from Washington University (Departments of Surgery, Medicine, and Pediatrics), Mid-America Transplant Services, and The Foundation for Barnes-Jewish Hospital.